
Grade 12 Biology - Unit One
The Chemicals of Life
All based off of the Nelson textbook "Grade 12 Biology 2003 edition."
Some Pointers:
- All chemicals of life are carbon based.
- Organic compounds are compounds that contain carbon. However, some carbon containing compounds such as carbides, carbonates, simple oxides of carbon (CO2), allotropes of carbon and cyanides are considered to be inorganic.
- The major classes of organic chemicals of biological importance include;
1) Carbohydrates
2) Lipids
3) Proteins
4) Nucleic Acids
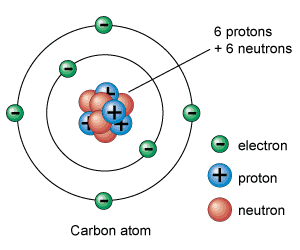
Carbon
Carbon plays a dominant role in the chemistry of life and is the fourth most abundant element in the universe by mass. It is a small, light element with four valence electrons located towards the vertices of a regular tetrahedron.
- Carbon atoms can attach to each other and form straight or branched chains and ringed structures. These structures act as the backbones of biological molecules.
- Molecules containing only hydrogen and carbon are called hydrocarbons. - The symmetrical geometry of the methane molecule causes carbon-hydrogen bonds to be non-polar.
- Functional groups may attach to the carbon backbone and gives the molecule its properties.
Examples of Functional groups and combinations are shown here. The compounds being added are functional groups forming other functional groups such as ester.

Biological Macromolecules
Macromolecules - Large molecules sometimes composed of a large number of repeating subunits.
The four major classes of macromolecules WITH their subunits are;
1) Carbohydrates - simple sugar (glucose)
2) Lipids - Glycerol and fatty acids
3) Proteins - Amino acids
4) Nucleic acids - Nucleotides
- The macromolecules listed above are known as polymers, which are made up of long chains of smaller subunits. These macromolecules may contain different subunits, but they are assembled and disassembled in the same way in the reactions listed below.
1) Condensation reaction (dehydration synthesis) - Creates a covalent bond between two interacting subunits by a removal of a hydrogen atom from a functional group of one subunit, and an -OH group from a functional group of another subunit. This H and -OH come together to form a water molecule. This reaction ABSORBS energy.
Anabolic reaction - Reactions that produce large molecules from smaller subunits. Ex. Condensation reaction.
2) Hydrolysis reaction - A catabolic reaction that breaks nutrient macromolecules down into subunits in the process of digestion. It is the reverse of condensation reactions and a water molecule is used to break apart a covalent bond holding subunits together by providing an H atom to one subunit and an -OH group to the other. These reactions RELEASE energy.
Catabolic reaction - Is when a water molecule is used to braek a covalent bond holding subunits together.
These two reactions require the assistance of special protein molecules, known as enzymes. Enzymes are discussed in another page of this website.